
Konjac glucomannan
Satiety and weight management
Glucomannan is a natural polysaccharide obtained from Amorphophallus konjac.
Konjac glucomannan is mainly used for weight loss and a claim can be made on its effect on satiety. Glucomannan has a high water retention capacity and when hydrated is highly viscous. It inflates the stomach and slows gastric emptying providing a sensation of satiety.
Kalys offers a wide range of glucomannan powders, can tailor glucomannan content, particle size and viscosity to customers’ needs or to match E425ii specifications.
Certified organic Konjac glucomannan is also available.![]()
It is available in France, Benelux and Spain.
Konjac gum and konjac glucomannan are high-molecular-weight polysaccharides obtained from the tubers of Amorphophallus konjac Koch, a traditional food plant in Asian countries that has been grown in China for more than 2000 years.
Glucomannan is cold-soluble and viscous. It is used mainly in dietary supplements for its satieting properties and in foods for its texturizing properties.
Kalys offers a wide range of konjac gums and konjac glucomannans in terms of particle size, apparent density, viscosity and concentration. They are also available with organic certification.
Konjac Glucomannane is available in France, Benelux and Spain.

Glucomannan structure
The main constituent of Konjac flour is the glucomannan, a polysaccharide of high molecular weight composed by D-manosa and by D-glucose in a molar relation from 1,6 to 1. He(she) presents ramifications every 50 ó 60 units and a group acetilo approximately every 19 units of saccharides.

Kalys' analytical methods
Glucomannan content: the recommended analytical method consists in measuring moisture and total dietary fiber content in konjac powder using the AOAC 985.29 method and assessing the quantity of insoluble fibers. By subtracting moisture from total dietary fiber and insoluble fiber in konjac powder, the total fiber and insoluble fiber contents by dry weight can be calculated. Glucomannan content on a dry weight basis is then calculated by subtracting insoluble fibers from total fiber content (by dry weight).
Viscosity: when comparing viscosity among different glucomannan ingredients, it is important to check that the percentage of konjac added to water and rehydration conditions (temperature, time) are the same. Most importantly, comparisons must be carried out using the same number of rotations per minute (rpm) because rehydrated glucomannan is sensitive to shear thinning. In other words, konjac viscosity decreases as shear increases. The diagram below shows that viscosity at 10 rpm is twice that at 30 rpm.
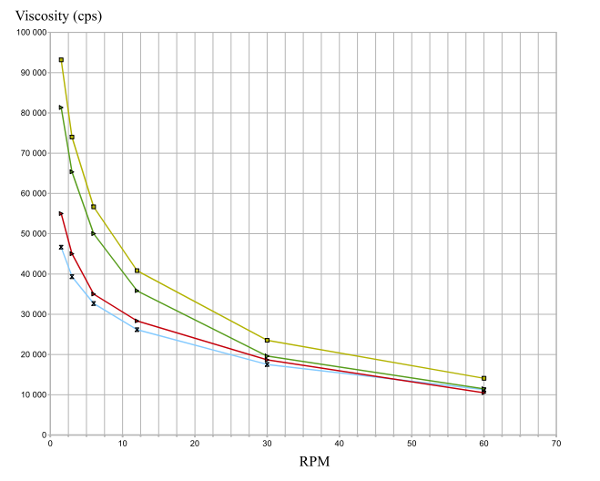
Viscosity of several konjac glucomannans from Kalys according to shear velocity
Satiety and weight management
Glucomannan can bind 15 to 20 times its weight in water and even up to 100 times for high-grade preparations. When hydrated, konjac glucomannan is highly viscous and slows gastric emptying providing a sensation of satiety. Konjac glucomannan also binds fats during the digestive process.
Konjac glucomannans have been used in China for a millennium to fill empty stomachs (the earliest mention dates from 206 BC in Shen Nong’s Herbal Classic) and satiate populations in times of food scarcity. It is now used in Europe for reducing food intake and body weight.

Cholesterol and postprandial glucose reduction
Glucomannan is a fiber as defined by Commission Directive 2008/100/EC. It lowers postprandial glucose and lipid levels and has prebiotic properties.
Konjac gum and konjac glucomannan are authorized as additives by Commission Regulation (EU) No. 231/2012 laying down specifications for food additives.
E425(i): Konjac Gum
E425(ii): Konjac Glucomannan
According to Regulation (EC) No. 1333/2008, food additives are substances that are not normally consumed as food itself but are added to food intentionally for a technological purpose. Therefore, konjac gum and konjac glucomannan, when used for nutritional purposes, ARE NOT used as food additives and the specific maximum level (SML) of 10 g per kg of food as laid down in Commission Regulation (EU) No 1129/2011 DOES NOT apply.

When used for its texturing properties, konjac gum and konjac glucomannan should be used at a SML of 10 g per kg of food individually or in combination.
Konjac glucomannan is listed in the 4th edition of the Food Chemical Codex (1996). Konjac glucomannan is published in FNP 52 Add 4 (1996), as prepared at the 46th JECFA.
The US Food and Drug Administration (FDA) has classified konjac flour as ‘generally recognized as safe’ (GRAS) (FDA, 1997).
Formulation in Dietary Supplements
Konjac glucomannan should preferably be presented in sachets or in hard gel capsules. Konjac glucomannan is not compressible.
In hard gel capsules, high glucomannan content and high density powders (40 mesh powders) should be preferred: 1 g glucomannan (or around 1.18 g konjac at 95% glucomannan by dry weight) can be packed in two hard gel capsules. Claims on weight management can be met with “only” two hard gel capsules before each meal but not three.
In sachets, 120 mesh powders should be preferred. Small particle size makes rehydration quicker and lowers the grainy sensation in the mouth.
Formulation in foods
Glucomannan is a viscous polysaccharide that is soluble in cold water. It has many possibilities in food technology, offering quick hydration and adaptable viscosity. Konjac is a natural alternative to galactomannan thickeners such as guar.
Konjac has a high water retention capacity: 1 g of konjac gum can bind between 15 and 20 g of water or 1.8 g of oil. High-grade preparations can bind up to 100 times their weight in water.
Synergistic effects can be obtained with various natural or modified food thickeners, even at low temperatures and low konjac ratios. Konjac forms cohesive thermoreversible gels, with runny-flexible to hard-brittle textures, depending on gum concentration. Konjac can be a partial substitute for other gums while maintaining strong gelling properties, useful for cost-effective formulations and more subtle variations in texture.
Konjac forms irreversible retort-stable gels in alkaline conditions.
Mixed with other ingredients, konjac can be used for the formulation of pasta, rice, or other low calorie, high fiber, and gluten-free applications such as sauces and creams, bakery and vegetarian, Asian, or pastry products. They are very interesting for dietetic dishes.
THE DIFFERENT RANGES OFFERED BY KALYS
Kalys offers several ranges of Konjac glucomannans that can be used in the formulation of diet, health and food products. A very wide range composed of several washed products is characterized by their:
- high rate of glucomannan: up to 95% on dry weight,
- granulometry: 40 to 120 mesh,
- viscosity: up to 40,000 Cps at 1% (25°C),
- high apparent density.
Within the organic range, there are unwashed organic certified products standardized at 80% glucomannan minimum on dry weight, they are available in different grain sizes and viscosities.
The unwashed products are natural or semi-refined. Coming from organic agriculture, konjac tubers do not undergo washing after drying and grinding. The powder is slightly more colorful, and its smell is characteristic of the plant.
In addition, Kalys offers washed organic certified konjac glucomannan. In order to obtain washed or refined Konjac glucomannan, Konjac tuber after slicing, drying, is grinding and then washing with a food-grade ethanol solution. This treatment eliminates non-fibrous residues and reaches a very interesting level of purity (EI> 95% high molecular weight of glucomannan on dry weight). The powder is clear, its taste and smell are neutral.
Nutrition claims
Glucomannan is a fiber according to the new Regulation (EC) No. 1169/2011, on the provision of food information to consumers.
Food products with added glucomannan can claim :
- to be a “source of fiber” if they contain at least 3 g glucomannan per 100 g or 1.5 g glucomannan per 100 kcal.
- to be “high in fiber” if they contain at least 6 g glucomannan per 100 g or 3 g glucomannan per 100 kcal.
Health claims
According to Commission Regulation (EU) No. 432/2012, two health claims can be made on foods and food supplements containing konjac glucomannan.

